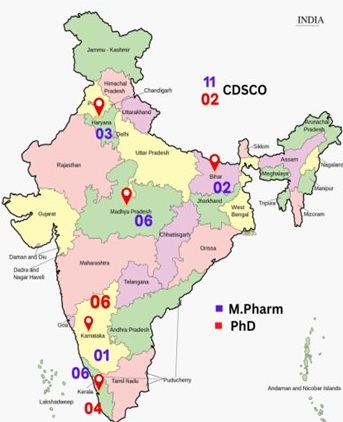
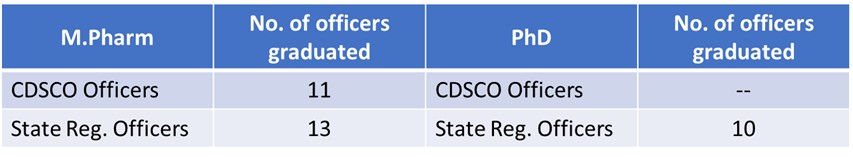
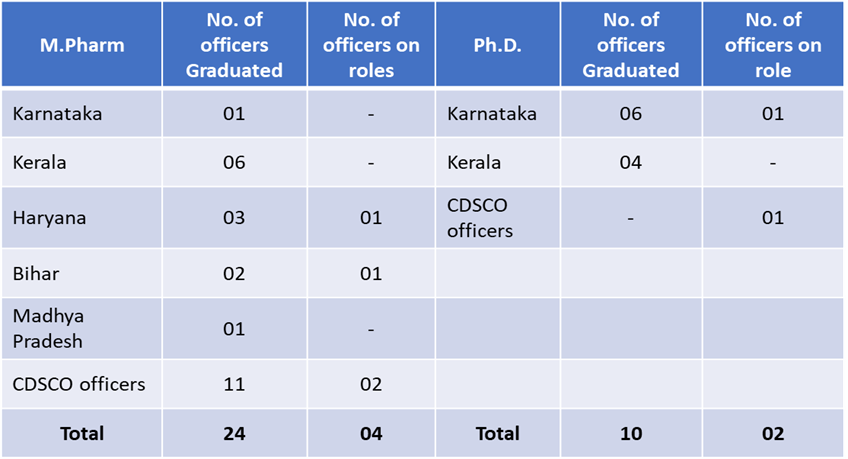
Centre of Excellence in Regulatory Sciences (CEReS)
Overview
Establishment Year: 30th November 2022.
Need for Establishment: To bridge the gap between SME's, Regulators and academics, the Centre of Excellence in Regulatory Sciences at JSS College of Pharmacy, Mysuru was established to address the regulatory issues and propel research in regulatory sciences.
The Centre of Excellence in Regulatory Sciences has made significant strides through eCTD training, workshops, and webinars, while fostering valuable collaborations with IKP and Biocon Academy. Our initiatives also include robust support to SMEs, reflecting our commitment to advancing regulatory science and supporting the broader industry ecosystem.


Area of Research Focus
- Development support to SMEs in collaboration with regulators
- Nurture regulatory science / educational programs
- Impart training to create and support regulatory submissions

Vision and Mission
- Vision: To improve and enhance skill set in regulatory science and utilize its resources to propel regulatory profession.
- Mission: To make available a pool of competent professionals in Pharmaceutical Regulatory Affairs with fundamental knowledge.

Key Objectives
- To create opportunities for budding regulatory professionals
- To build collaborative interactions to foster regulatory science communications
- To impart training to create and submit regulatory dossiers
- To provide a focal point for knowledge management, with the ability to capture new knowledge and best practices from the healthcare sector
Inaguration
- Date: 30th November 2022
- Institution/Department: Dept. of Pharmaceutics, JSS College of Pharmacy, Mysuru
- Reporting Period: 30th November 2022 - 11th September 2024
US-FDA India Office team
Dr. Sarah McMullen, Country Director – India Office
Mr. Gregory Smith, International Relation Specialist – Drugs
Dr. Sudheendra Kulkarni, Medical Product Safety Coordinator – BIMO, Devices
Mr. Dhruv Shah Medical Product Safety Coordinator – Drugs
Key Activities and Achievements
Collaborations
- National: IKP Knowledge Park, Bengaluru
- National: Biocon Academy, Bengaluru
- International: CyVidia, Chicago, USA
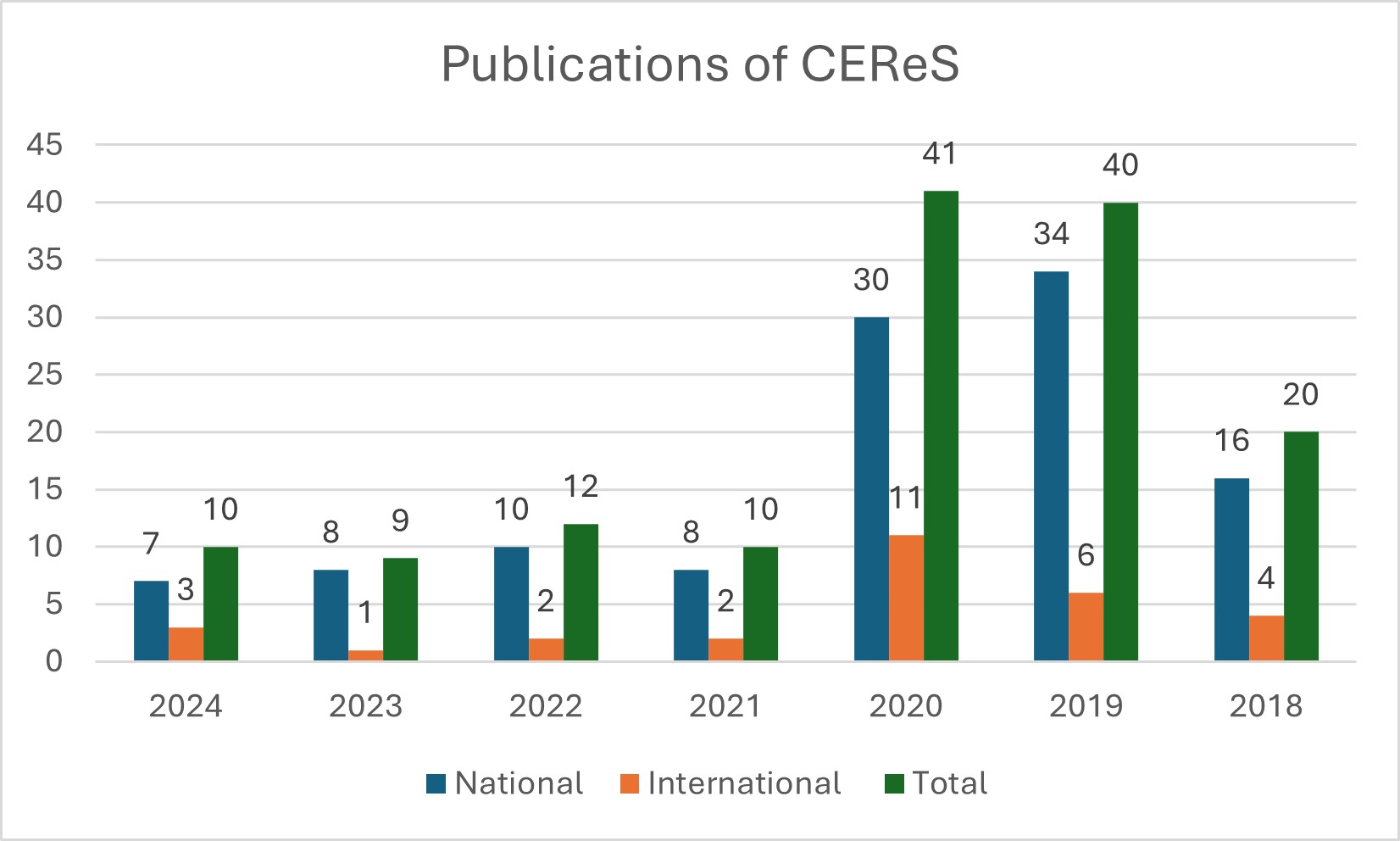
Number of publications in peer-reviewed journals: 140 and Conference presentations: 46
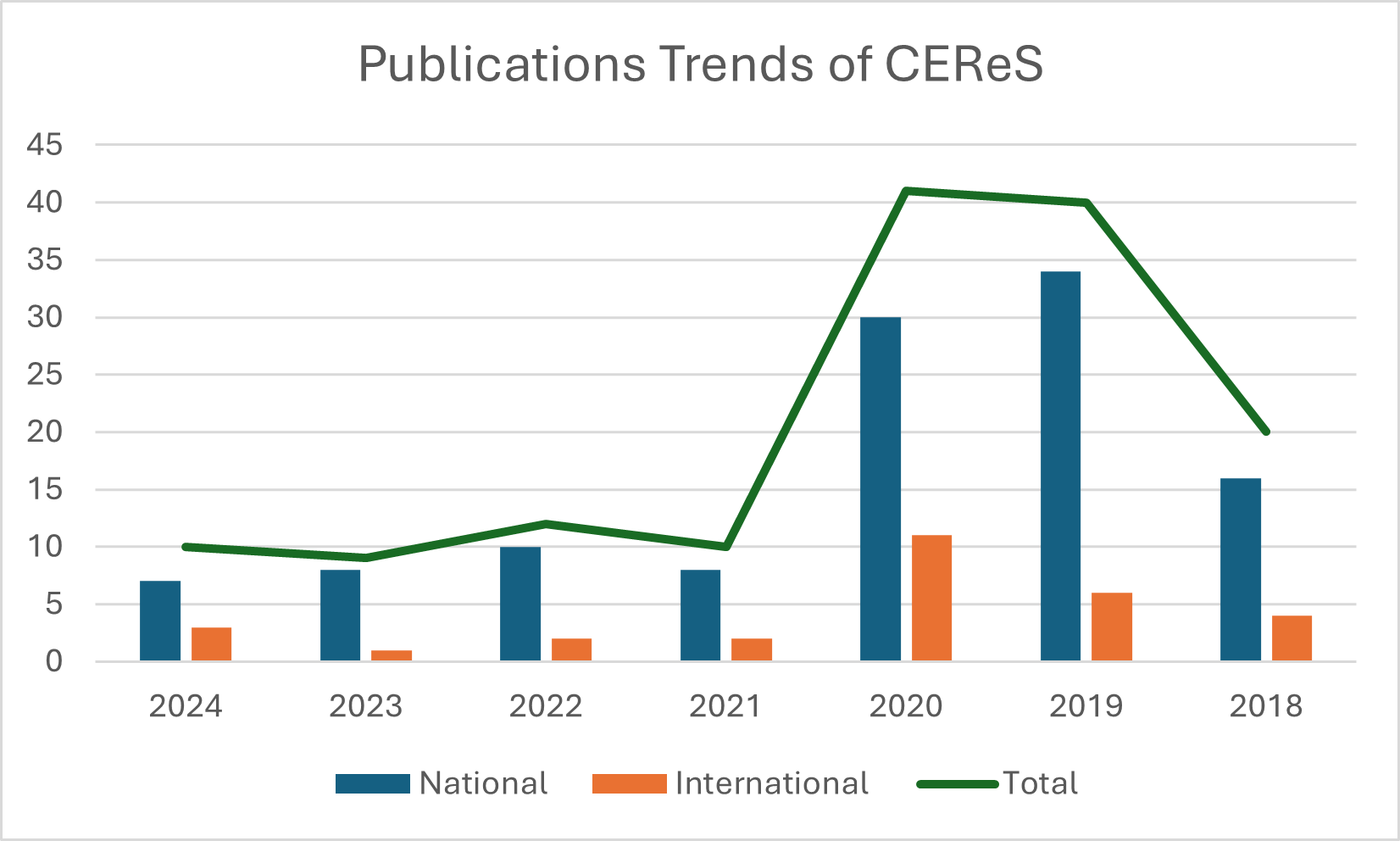
Infrastructure and Resources
Infrastructure facilities: Regulatory Studio
- Regulatory Affairs Lab with 54 workstations, IT enabled facilities
- PharmaReady® Software for eCTD submissions
- 14 desktop computers with high-speed internet
Advisors

Dr. B Suresh
Pro-Chancellor
JSS AHER, Mysuru

Dr. Surinder Singh
Vice Chancellor
Ex-DCGI, Govt. of India
Ex-Director, NIB
JSS AHER, Mysuru

Dr. Bangaru Rajan
Professor
Reg. Affairs & Ex-JDC, CDSCO, New Delhi
JSS AHER, Mysuru

Dr. B Suresh
Pro-Chancellor
JSS AHER, Mysuru

Dr. Surinder Singh
Vice Chancellor
Ex-DCGI, Govt. of India
Ex-Director, NIB
JSS AHER, Mysuru

Dr. Bangaru Rajan
Professor
Reg. Affairs & Ex-JDC, CDSCO, New Delhi
JSS AHER, Mysuru
Faculty

Dr. T M Pramod Kumar
Principal and Professor

Dr. Bangaru Rajan
Professor

Dr. Balamuralidhara V
Associate Prof & HoD

Dr. M P Venkatesh
Associate Professor

Dr. G S Meghana
Lecturer
Centre of Excellence in Regulatory Affairs (CEReS) Expert Panel
Following drug regulatory officers from CDSCO and state regulatory officers are on the rolls as Expert in the Panel to support CEReS and its activities.
| Sl. No | Name of the Expert | Affiliation |
|---|---|---|
| 1 | Dr. G. Selvaraj | Director of Drugs control TN (Retd.) |
| 2 | Mr. M.Bhaskaran | Director of Drugs control TN (Retd.) |
| 3 | Dr. Sateesh | Director of Drugs control Kerala (Retd.) |
| 4 | Mr. M.N.Sridhar | Joint Director of Drugs Control, TN |
| 5 | Dr. Jagashetty | Drugs Controller, Karnataka (Retd.) |
| 6 | Dr. Amaresh Tumbagi | Additional Drugs Controller, Karnataka (Retd.) |
| 7 | Mr. Om Prakash Sadwani | Joint Commissioner FDA Maharashtra (Retd.) |
| 8 | Mr. Ananda Das | Drugs controller, Odisha (Retd.) |
| 9 | Dr. Mandal | Deputy Drugs Controller, West Bengal (Retd.) |
| 10 | Mr. Lalith Goel | Deputy Drugs Controller, Haryana |
| 11 | Mr. Arivzhagan | Deputy Drugs Controller, Delhi (Retd.) |
| 12 | Dr. Atul Nasa | Drugs Controller, Delhi (Retd.) |
| 13 | Dr. Goswami | Drugs controller Tiripura (Retd.) |
| 14 | Mrs. Lotika | Drugs Controller, J&K |
| 15 | Mr. Salem Veljee | Commissioner FDA Goa (Retd.) |
| 16 | Mrs. Jothi Sardesai | CommissionerFDA, Goa |
| 17 | Mr. Mahapatra | Drugs controller Odisha (Retd.) |
| 18 | Mr. Lalsawma Pachuau | Drug controller Mizoram |
| 19 | Dr. Vijay | Joint Commissioner, FDA Gujarat (Retd.) |
| 20 | Dr. Sella Senthil, | Assistant Drugs Controller, CDSCO, New Delhi |
| 21 | Mr. R.K.Sinha | Drugs Controller, Bihar |
| 22 | Mr. A.K.Jain | Drugs Controller, UP |
| 23 | Dr. Ananthakrishnan | Deputy drugs Controller, Puduchery |
| 24 | Mr. Hridyananda Mahanta | Ex. Drug Controller, Assam |
| 25 | Mrs. Shanthy Gunasekaran | JDC (I) (Retd.) CDSCO |
| 26 | Dr. Manivannan | Deputy Drugs Controller, CDSCO, Bangalore Zone |
| 27 | Dr. D.Roy | Deputy Drugs controller (I),CDSCO (Retd.) |
| 28 | Dr. Ramkishan | Deputy Drugs Controller (I), Hyderabad |
| 29 | Dr. Adeshra | EX Commissioner, FDA, Gujarat |
The board of Expert committee of CEReS will give their advice/expertise in taking forward the purpose of CEReS
- By identifying the upcoming newer areas in Pharmaceutical sciences to initiate the research in those areas
- By giving solutions to the problems encountered by the SMEs to comply with the current GXPs
- By collaborating with the team members of Industry and Institutions to start the Newer projects
- Be an expert/resource person in the Training Programs / workshops/ seminars/ conferences organized for the benefit of Students, staffs and SMEs
- To help in the preparation in Simplified Language through descriptive documents, the current Regulatory Requirements for manufacture of APIs, Finished Formulations, Biologicals, Medical Devices, IVDs and other New Drug Delivery Systems
Global Regulatory Affairs (GRA) Certificate program with Biocon Academy, Bengaluru [JSS AHER, Mysuru is the Knowledge Partner]
Biocon Academy, Bengaluru offers high quality innovative programs focussed on the Business of Biosciences with the aim to empower experienced professionals from the field of Life sciences and to develop fresh biotech graduates into Biosciences and other related professionals. They have partnered with JSS Academy of Higher Education and Research (JSS AHER) as a Knowledge Partner for conduct and delivery of Global Regulatory Affairs (GRA) program.
JSS College of Pharmacy, Mysuru, a constituent college of JSS AHER is collaborating partner with Biocon Academy (BA) for the Joint Certificate Program in Global Regulatory Affairs (GRA) titled “Biocon-JSSAHER Certificate program in Global Regulatory Affairs”. For the GRA program, JSS AHER, Mysuru is the knowledge partner and the faculty of JSS College of Pharmacy, Mysuru would deliver didactic lectures online (for 16 weeks) and impart hands-on- training (5 days) at JSSCP Mysuru to the enrolled candidates. After the successful completion of the program, candidates are awarded joint certificates from Biocon Academy and JSS AHER.
The GRA program is a 16-weeks course with 6 subject areas [Introductory/ Preparatory Regulatory Affairs, Small Molecule Regulatory Affairs (Drugs), Large Molecule Regulatory Affairs (Biologics), Clinical Regulatory Affairs, Medical Devices Regulatory Affairs, Documentation and Regulatory Approvals] relevant to global/ industrial regulatory requirements. The syllabus and content were proposed by JSSAHER, reviewed, and accepted by both parties.
Monitoring of the program: The GRA program was monitored regularly by means of monthly meetings between coordinators of Biocon Academy and JSSCP, Mysuru. Updates of the program was discussed, and any issues related was also discussed for the smooth conduct of the program. The assessment scores of the evaluation were promptly shared with Biocon Academy which gave them a platform to follow up with the students.
Hands on Training: 5 days Hands on training will be conducted at the end of the program
The following activities were conducted during the training at JSSCP Mysuru.
- eCTD compilation and submission
- electronic submissions by employee shadowing
- filling 356h forms
- CDSCO SUGAM portal
- training on pharmacovigilance and clinical trial activities
Evidence of Success: It is clearly evident that GRA students of all 3 batches were successfully placed in reputed pharmaceutical/ biopharmaceutical organizations in good roles.
| Sl. No. | Batch Number | Activity dates | No. of students |
|---|---|---|---|
| 1. | GRA Batch 1 |
Program: 30th Aug 2021 to 24th Dec 2021 Hands-on-training: 3rd Jan to 7th Jan 2022 |
19 |
| 2. | GRA Batch 2 |
Program: 10th Feb to 26th May 2023 Hands-on-training: 29th May to 2nd June 2023 |
18 |
| 3. | GRA Batch 3 |
Program: 12th February to 29th May 2024 Hands-on-training: 2nd June to 7th June 2024 |
19 |
Faculty of JSS College of Pharmacy, Mysuru for Global Regulatory Affairs Program:
| Sl. No. | Faculty | Designation |
|---|---|---|
| 1. | Dr K Bangarurajan | Professor |
| 2. | Dr Balamuralidhara V | Associate Professor |
| 3. | Dr H V Gangadharappa | Associate Professor |
| 4. | Dr. M.P. Venkatesh | Associate Professor & Coordinator - GRA |
| 5. | Dr Gowrav M P | Assistant Professor |
| 6. | Dr Hemant Kumar S | Assistant Professor |
| 7. | Dr Shailesh T | Lecturer |
| 8. | Dr. G.S. Meghana | Lecturer |
Challenges and Opportunities
Challenges
Regulatory sciences, which encompass the development, implementation, and evaluation of policies and procedures to ensure the safety and efficacy of medical products face variety of challenges like
- Keeping up with Rapid Technological Advances
- Global Harmonization
- Complexity of Data
- Resource Constraints
- Regulatory Flexibility and Adaptability
Addressing these challenges requires a collaborative approach, involving regulators, industry experts, researchers, academia and other stakeholders to ensure that regulatory practices evolve in line with scientific advancements and public health needs.
Opportunities
Regulatory sciences offer several opportunities to enhance public health, facilitate innovation, and improve the efficiency and effectiveness of regulatory processes.
- Regulatory Science Education and Training: Expanding educational and training programs in regulatory sciences can build a skilled workforce capable of addressing current and future challenges. These programs can also promote interdisciplinary collaboration and innovation in regulatory practices.
- Adaptive Regulatory Pathways: Developing and implementing adaptive regulatory pathways can accelerate the approval of innovative products
- Integration of Advanced Technologies: The incorporation of technologies such as artificial intelligence (AI), machine learning, and big data analytics can significantly enhance the ability to predict safety issues, streamline regulatory processes, and analyze complex data sets.
Future Directions
Upcoming Projects/Initiatives
- Centre of Excellence in Regulatory Sciences is actively engaged in organizing seminars/ symposium/ workshop/ training programs in regulatory sciences.
- As a part of continuing educational activities, CEReS is planning to conduct 2 webinars/ seminars/ workshops in this calendar year.
Collaborative Efforts Planned
Collaborations with National and International Regulatory Agencies:
- CEReS is actively working to collaborate with United States -Food and Drug Administration (US-FDA) to have an MoU/ MoI.
- CEReS is focusing on strengthening our collaboration efforts with CDSCO, New Delhi to enhance regulatory processes and advance our mutual goals.
- Centre of Excellence in Regulatory Sciences (CEReS) is expanding its collaborative activities with SMEs (Medical devices, drugs and cosmetic industries) to foster research and regulatory submissions.
- Establishment of a robust industry-academia collaborations can bridge the gap existing in regulatory sciences and make available talented pool of regulatory professionals.
Capacity Building Programs:
- Establishment of a robust industry-academia collaborations can bridge the gap existing in regulatory sciences and make available talented pool of regulatory professionals.
- Centre of Excellence in Regulatory Sciences (CEReS) is expanding its collaborative activities with SMEs (Medical devices, drugs and cosmetic industries) to foster research and regulatory submissions.
Anticipated Funding Applications:
Given our plans for significant expansion in our activities, we wish to seek funding from national and international agencies to support our efforts.