JSS College of Pharmacy, Mysore
Webinar on Recent updates on IVD Regulation and Business and Employment Opportunities in IVDs
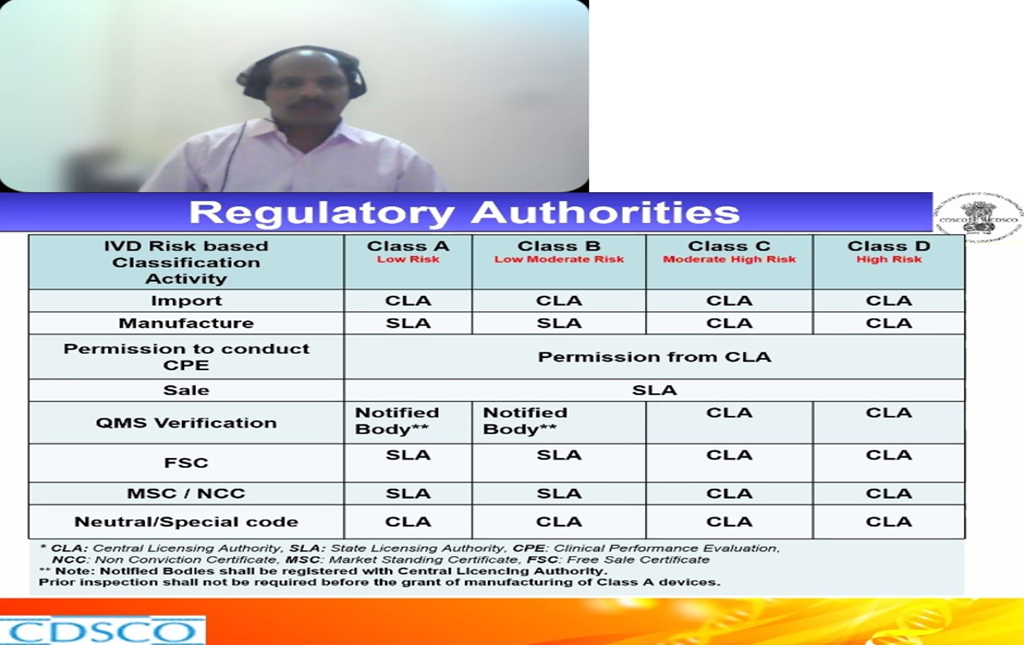
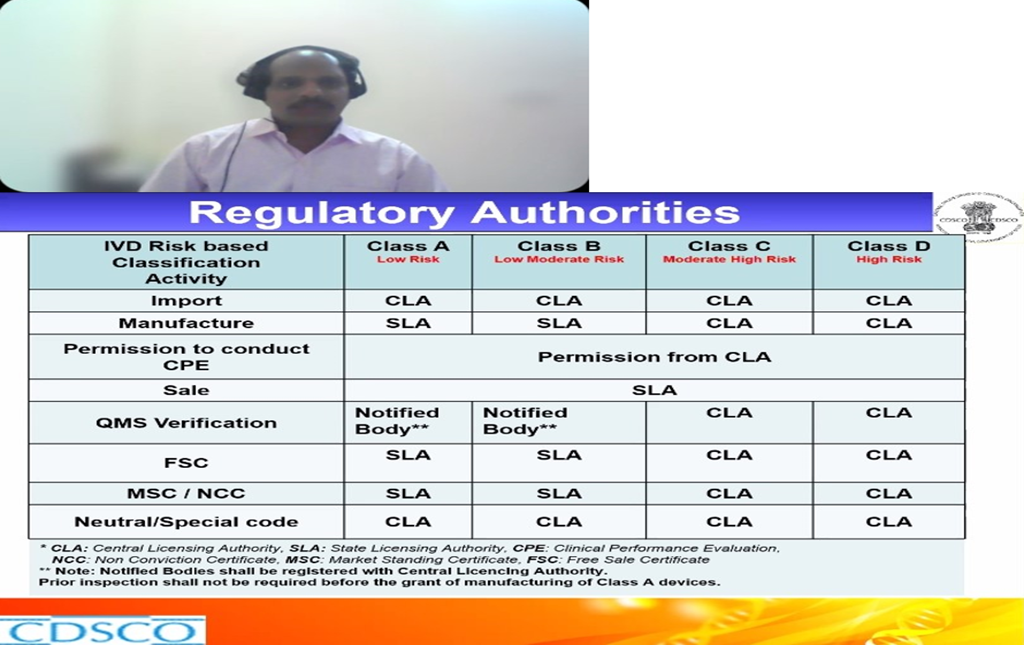
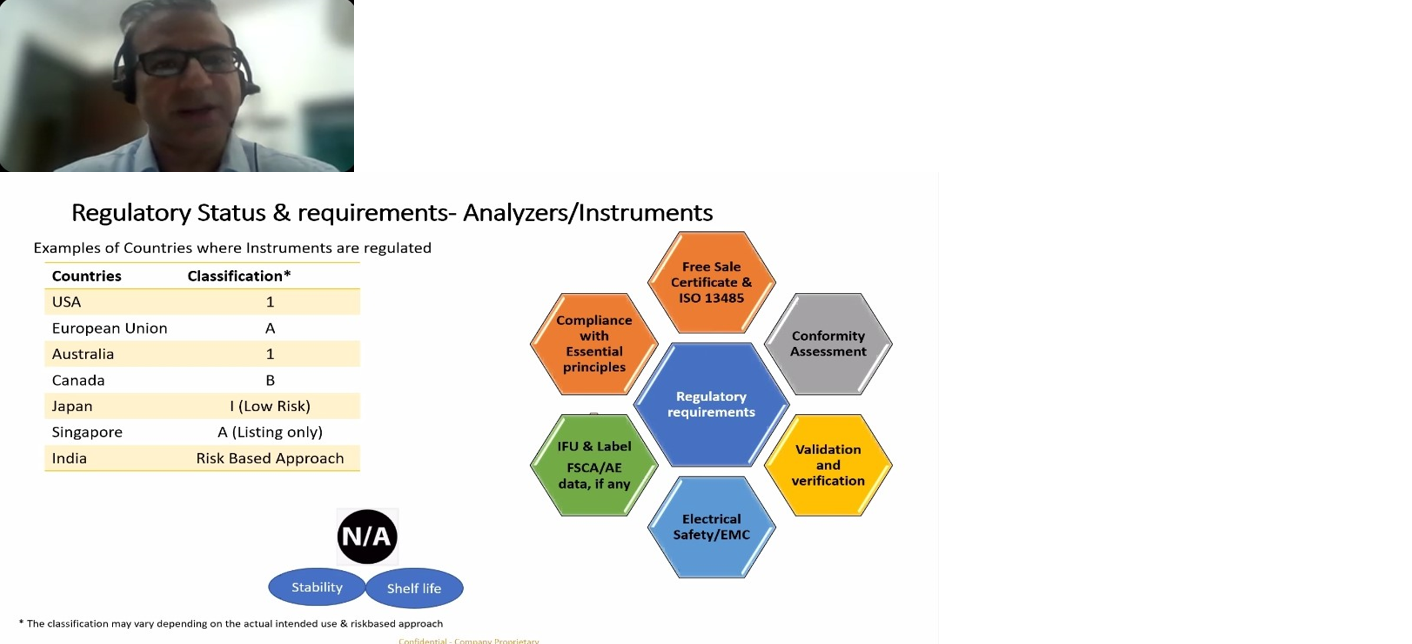
Dr. Sella Senthil, ADC(I), CDSCO was the first speaker of the webinar session spoke about the topic “Indian IVD Regulatory Landscape”. He discussed about what are IVD’s, definitions, what are they used for and how they differ from medical devices and Drugs. How IVD’s are regulated under Drugs and Cosmetics Act 1940 and rules 1945 and what are notified and un notified IVD’s and what are the Regulatory authorities in India. What are the roles of state licensing authority and central licensing authorities in IVD. He further gave a talk on how IVD’s are classified in India, what does it mean by Low risk-Class-A, Low moderate risk Class- B, Moderate high-risk Class-C, High risk Class-D IVD’s and what are they used for. What are the licensing authorities in India and how different classes of IVD’s are licensed like for Class A, B, C, D. Where and how IVD’s are evaluated in India, what are. He discussed about what are new IVD’s and approved IVD’s and their definitions. He further explained the specifications for each class of IVD’s, what are the different central institutions and why outsourcing for evaluation is done in their evaluation, where to register and what are the checklists for the submission. How to access the CDSCO website for getting information on the banned, approved IVD’s. He gave us a brief note on the IVD regulatory pathway in India, documents to be submitted, approval process through online portal, approval process for Class- A/B/C/D manufacturing license, approval process for post approval changes and retention of license. He also discussed on the import and manufacture license for the IVD, how many years it is valid and what is the retention time.
Read More