JSS College of Pharmacy, Mysore
webinar on Fixed Dose Combinations and need for FDCs in Patients Health Care

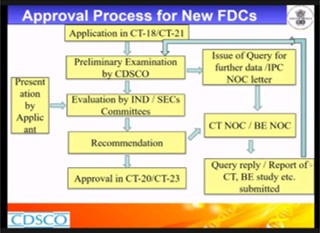
Mr. K Narendran, Assistant Drug Controller (ADC), was the first speaker of the webinar. He started his talk by giving an overview of “Regulations on Fixed-dose combinations in India”. He discussed about the categories of FDCs, fees payable for review of FDC application and approval process of FDCs. Further he elaborated on various regulatory aspects including submission requirements and the committees for approval process of new FDCs.
Read More