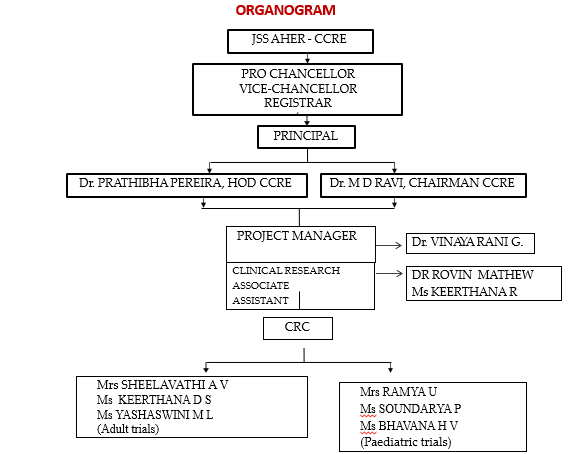
Introduction
Welcome To Center for Clinical Research Excellence

Centre for Clinical Research Excellence (CCRE), at JSS AHER was approved as a collegium of Clinical Development Services Agency (CDSA) - A DBT, Ministry of Science and Technology, Government of India initiative to facilitate the development of healthcare products for public health system in 2015 (http://www.cdsaindia.in).The department started its operations in clinical research training in 2016, and we are now fully set up and started with our clinical trials related operations.
The CCRE received the BIRAC project entitled "Capacity building of Infrastructure and human resource for conducting COVID Vaccine clinical trials and Immunogenicity studies as per GCP guidelines" from Department of Biotechnology, under Mission COVID Suraksha, New Delhi on 19.04.2021.
Additionally, the center successfully completed a BIRAC audit in 2021 with no NON-COMPLIANCE, further validating its robust governance, financial management, and operational systems.
A major academic milestone was achieved with the establishment of the Department of Clinical Research and initiation of the Master of Science (MSc) program in Clinical Research. To further strengthen academic innovation and outreach, the department will be launching the MSc Clinical Research program in Asynchronous Learning Mode, ensuring wider accessibility and flexibility while maintaining academic rigor.
CCRE received Intent from ICMR in 16.10.2024, enhancing its national academic recognition.
In the area of regulatory compliance and quality excellence, CCRE successfully underwent DCGI inspections in July 2019 and Dec 2025, with no non-compliances observed, demonstrating strong adherence to Good Clinical Practice (GCP) and regulatory requirements.
Collectively, these outcomes represent one of the most significant achievements of CCRE, reflecting the center's unwavering commitment to regulatory excellence and its dedication to maintaining the highest levels of quality and integrity in clinical research.