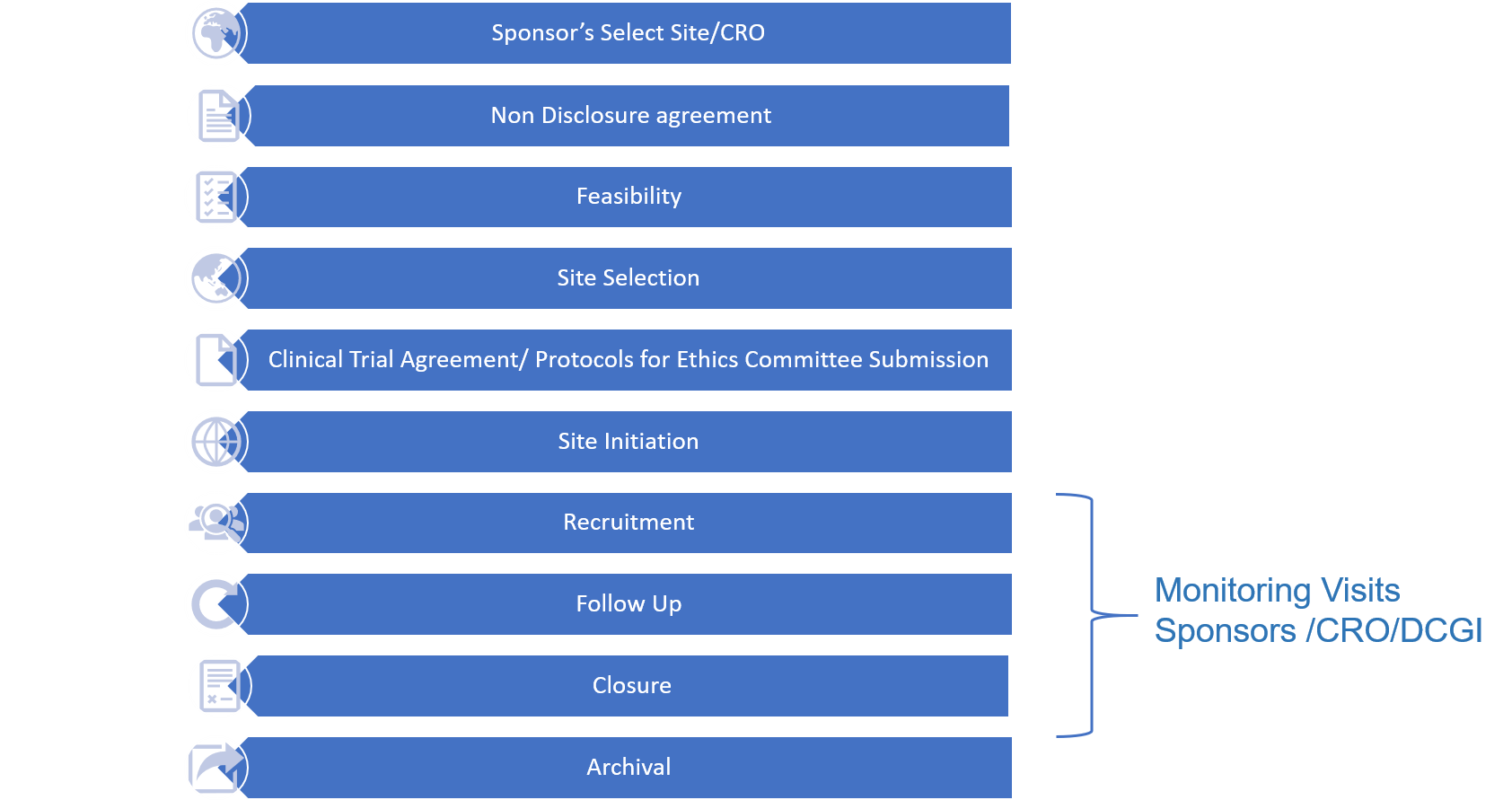
Introduction of Clinical Trials
Clinical Trials, also known as clinical studies, test potential treatments in human volunteers to see whether they should be approved for wider use in the general population. A treatment could be a drug, medical device, or biologic, such as a vaccine, blood product, or gene therapy. Potential treatments, however, must be studied in laboratory animals first to determine potential toxicity before they can be tried in people. Treatments having acceptable safety profiles and showing the most promise are then moved into clinical Trials.
Although "new" may imply "better," it is not known whether the potential medical treatment offers benefit to patients until clinical research on that treatment is complete. Clinical Trials are an integral part of new product discovery and development and are required by the Food and Drug Administration before a new product can be brought to the market.
The FDA is committed to protecting the participants of clinical Trials, as well as providing reliable information to those interested in participating. Recently, unethical behavior on the part of some researchers has shaken the public trust and prompted the federal government to establish regulations and guidelines for clinical research to protect participants from unreasonable risks.
Although efforts are made to control risks to clinical Trial participants, some risk may be unavoidable because of the uncertainty inherent in clinical research involving new medical products. It's important, therefore, that people make their decision to participate in a clinical Trial only after they have a full understanding of the entire process and the risks that may be involved.