About Institutional Ethics Committee
The purpose of the IEC, JSSMC is to protect human subjects through ensuring highest ethical standards and conduct in all research carried out under the auspices of JSSAHER.
Institutional Ethics Committee, JSSMC is Re-Registered vide No. ECR/387/ Inst /KA/2013/ RR-22 issued under Rule 122DD of the Drugs & Cosmetics Rules 1945 by the Joint Drugs Controller (I) & Licensing Authority, Govt. of India , Ministry of Health & Family Welfare, Directorate General of Health Services, Office of Drugs Controller General (India), Central Drugs Standard Control Organization (CDSCO).

Institutional Ethics Committee, JSSMC also has the registration of the Ethics Committee relating to Biomedical and Health Research with the National Ethics Committee Registry for Biomedical and Health Research (NECRBHR), Department of Health Research (DHR), Certificate vide No: NECRBHR: DHR Reg No: EC/NEW/INST/2021/2254.
Institutional Ethics Committee, JSSMC is accreditated by NABH since 2018 vide Certificate No. E-CT- 2018-0018. Presently re-accreditated from April 2024-April 2027.
The Principal, JSS Medical College is the authority under which IEC, JSSMC is established and is functioning. IEC, JSS MC is Independent and competent in its functioning and decision making.
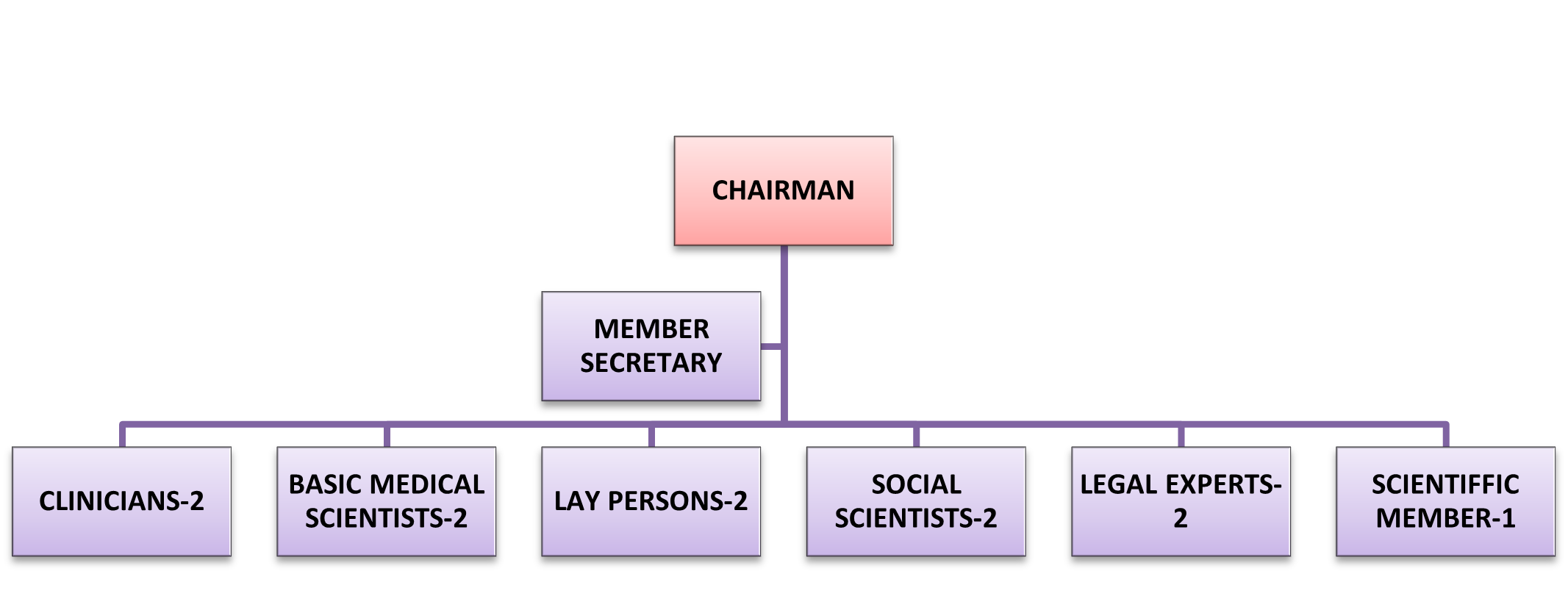
The IEC is multidisciplinary and multisectoral in composition. The IEC, JSSMC is headed by a Chairperson and supported by 12 other members including the Member Secretary.
IEC has adopted Standard operating procedures (SOPs) to ensure the protection of the rights, safety and welfare of human participants in clinical, biomedical and health research.
Institutional Ethics Committee of JSS Medical College, shall function according to the rules and regulations envisaged in the International Conference of Harmonization - Good Clinical Practice (ICH-GCP) guidelines, Indian GCP guidelines, CDSCO- DCGI New Drugs and Clinical Trial Rules 2019, ICMR Ethical Guidelines for Bio-Medical Research on human participants 2017 and any other applicable guidelines and also in accordance with SOP of IEC, JSS MC , Mysore.